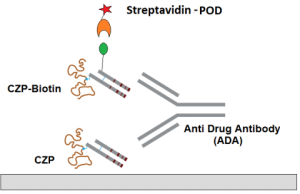
Enzyme immunoassay for the quantitative and qualitative determination of free antibodies to Certolizumab Pegol (Cimzia®) in serum and plasma.
The solid phase (MTP) is coated by the drug Certolizumab Pegol. Due to the assay design this test measures the free antibodies which are not bound to Certolizumab Pegol. Results could be expressed in both qualitative and quantitative manner (nanogram per mililiter-ng/mL).
Reference: IG-BB109
Barcode: 8682754930264
Legal Status: CE-Mark
Intended Use: This kit has been developed for the detection of anti-drug antibodies in research and diagnostic uses. It is suitable for Therapeutical Drug Monitoring (TDM) purposes.
Assay Design:
Related Documents
Quality Control Certificates
Cimzia® is a trademark of UCB.