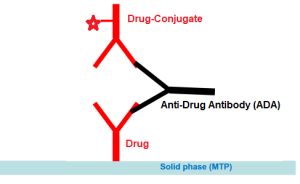
Enzyme immunoassay for the quantitative and qualitative determination of free antibodies to Omalizumab (Xolair®) in serum and plasma.
The solid phase (MTP) is coated by the drug Omalizumab. Due to the assay design this test measures the free antibodies which are not bound to Omalizumab. Results could be expressed in both qualitative and quantitative manner (nanogram per mililiter-ng/mL).
Reference: IG-BB111
Barcode: 8682754930288
Legal Status: CE-Mark
Intended Use: This kit has been developed for the detection of anti-drug antibodies in research and diagnostic uses. It is suitable for Therapeutical Drug Monitoring (TDM) purposes.
Assay Design:
Xolair® is a trademark of Genentech Inc.