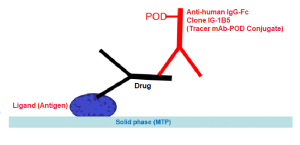
Enzyme immunoassay for the quantitative determination of free Infliximab (Remicade®, Remsima®) in serum and plasma.
The solid phase (MTP) is coated by the target molecule, in this case TNF-alpha.
Reference: IG-AA101
Barcode: 8682754930028
Legal Status: CE-Mark
Intended Use: This kit has been developed for the measurement of drug levels in research, diagnostic and biosimilar uses. It is suitable for Therapeutical Drug Monitoring (TDM) purposes.
Assay Design:
Related Documents
Quality Control Certificates
Remicade® is a trademark of Janssen Biotech, Inc./Merck & Co.
Remsima® is a trademark of CellTrion Inc.