Rituximab ELISA (mAb-based)
Enzyme immunoassay for the specific and quantitative determination of free Rituximab (Rituxan®, Mabthera®) in serum and plasma.
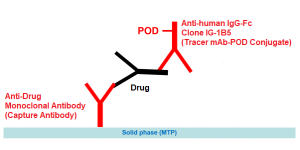
The solid phase (MTP) is coated by a highly specific monoclonal antibody directed against Rituximab. Therefore any cross reactivity to the other therapeutical monoclonal antibodies is excluded.
Reference: IG-AB106
Barcode: 8682754930189
Legal Status: CE-Mark
Intended Use: This kit has been developed for the measurement of drug levels in research and diagnostic uses. It is suitable for Therapeutical Drug Monitoring (TDM) purposes.
Assay Design:
Related Documents
Quality Control Certificates
Rituxan® and Mabthera® are trademarks of Biogen Idec/Genentech Inc.